The first-ever drug created specifically to combat postpartum depression was approved by the US. Zulresso infusion is a new and effective treatment for postpartum depression.
 Stepped Care Treatment Of Postpartum Depression Women S Health Issues
Stepped Care Treatment Of Postpartum Depression Women S Health Issues
Relevance to Patient Care and Clinical Practice.

Iv infusion for postpartum depression. But on Tuesday the Food and Drug Administration approved the first drug specifically developed for postpartum depression called brexanolone or Zulresso. The findings of the review show that brexanolone administered via IV infusion is both an effective and a fairly safe option for the treatment of PPD. Food and Drug Administration.
Brexanolone is novel because it. The FDAs approval of this. On March 19 2019 The FDA approved Zulresso brexanolone as the first drug treatment specifically for postpartum depression PPD.
Although more research needs to be done this makes IV ketamine therapy an exciting treatment option for postpartum depression where getting better quickly is what every mom hopes for and where restoring the health of the mother. 61 A potential limitation of brexanolone is the estimated cost of 34000 for one continuous infusion. The Drug Enforcement Administration DEA has placed Zulresso brexanolone.
ZULRESSO is a prescription medicine used to treat Postpartum Depression in adults. Social Sharing Postpartum depression is a. WEDNESDAY March 20 2019 HealthDay News -- Postpartum depression is a common and often devastating condition for new mothers but the US.
In March 2019 the FDA approved Zulresso brexanolone which is an intravenous IV infusion for treating postpartum depression PPDZulresso is the first drug approved by the FDA that is specifically for PPD. Zulresso is administered as a one time infusion. By Will Boggs MD.
ZULRESSO can cause serious side effects including. Brexanolone represents a novel approach to the treatment of depressionTraditional antidepressants or Selective Serotonin Reuptake Inhibitors SSRIs like Zoloft. IV ketamine is an extremely rapid effective treatment for depression and 75-80 of patients respond quickly and dramatically to very low dose IV infusions.
The financial impact of PPD can be substantial and attributable to a multitude of factors including but not limited to. Sage Therapeutics a treatment for. There are several antidepressants currently used to treat PPD.
1 Postpartum depression is a serious condition that can interfere with a mothers ability to take care of herself and her family. Food and Drug Administration on. Will a promising new drug Zulresso become affordable and accessible enough to help them.
The rapid onset of action of brexanolone may offer a quicker relief of. NEW YORKA 60-hour infusion of brexanolone significantly improves symptoms in women with postpartum depression according to results from two phase 3 trials. IV ketamine has a 70 to 80 success rate depending on which study you read in decreasing a patients symptoms by at least half within the first one or two infusions.
Postpartum depression hits low-income women especially hard. Brexanolone Infusion Rapidly Relieves Postpartum Depression. And increased hospital costs.
At Lone Star Infusion we have been offering treatment for new moms with postpartum depression since 2015. Severe cases can lead to thoughts of harming yourself or even your baby. Food and Drug Administration FDA on Tuesday.
These side effects need to be balanced against the considerable benefits of this treatment. However it involves potentially serious risks severe sedation or loss of consciousness. Other common side effects of Zulresso include sleepiness headache dizziness vertigo dry mouth and flushing.
Lost productivity due to depression. But its an expensive infusion and requires a stay in. First postpartum depression therapy an IV drug approved by US.
Approves First Drug for Postpartum Depression The medication works quickly within 48 hours. ZULRESSO may cause you to feel very sleepy excessive sedation or pass out loss of consciousness. Postpartum mother and child morbidity and mortality.
Excessive sedation and sudden loss of consciousness. However this is the first with FDA approval for this indication. Food and Drug Administration today approved Zulresso brexanolone injection for intravenous IV use for the treatment of postpartum depression.
 Group Based Interventions For Postpartum Depression An Integrative Review And Conceptual Model Archives Of Psychiatric Nursing
Group Based Interventions For Postpartum Depression An Integrative Review And Conceptual Model Archives Of Psychiatric Nursing
 Fda Approves Zulresso For First Treatment For Postpartum Depression
Fda Approves Zulresso For First Treatment For Postpartum Depression
 Brexanolone Sage 547 Injection In Post Partum Depression A Randomised Controlled Trial The Lancet
Brexanolone Sage 547 Injection In Post Partum Depression A Randomised Controlled Trial The Lancet
 Pharmacological Treatment For Postpartum Depression Download Table
Pharmacological Treatment For Postpartum Depression Download Table
 New Medication Could Help Moms With Postpartum Depression Abc News
New Medication Could Help Moms With Postpartum Depression Abc News
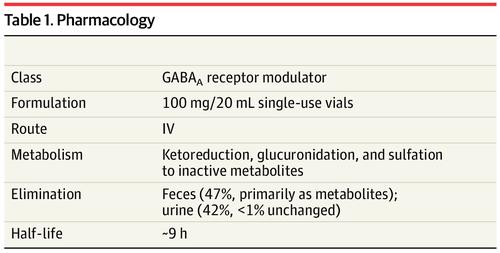 Brexanolone Zulresso For Postpartum Depression Jama X Mol
Brexanolone Zulresso For Postpartum Depression Jama X Mol
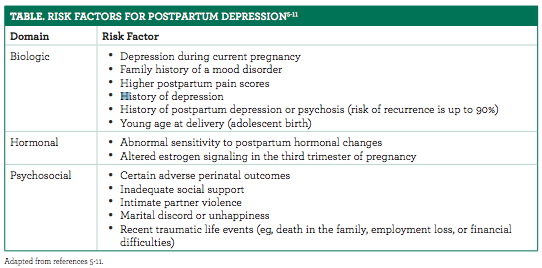 Postpartum Depression Is Not Just Baby Blues
Postpartum Depression Is Not Just Baby Blues
 Mitigating The Effect Of Persistent Postnatal Depression On Child Outcomes Through An Intervention To Treat Depression And Improve Parenting A Randomised Controlled Trial The Lancet Psychiatry
Mitigating The Effect Of Persistent Postnatal Depression On Child Outcomes Through An Intervention To Treat Depression And Improve Parenting A Randomised Controlled Trial The Lancet Psychiatry
 Efficacy Of Interpersonal Psychotherapy For Postpartum Depression O
Efficacy Of Interpersonal Psychotherapy For Postpartum Depression O
 Fda Approves First Postpartum Depression Drug Cnn
Fda Approves First Postpartum Depression Drug Cnn
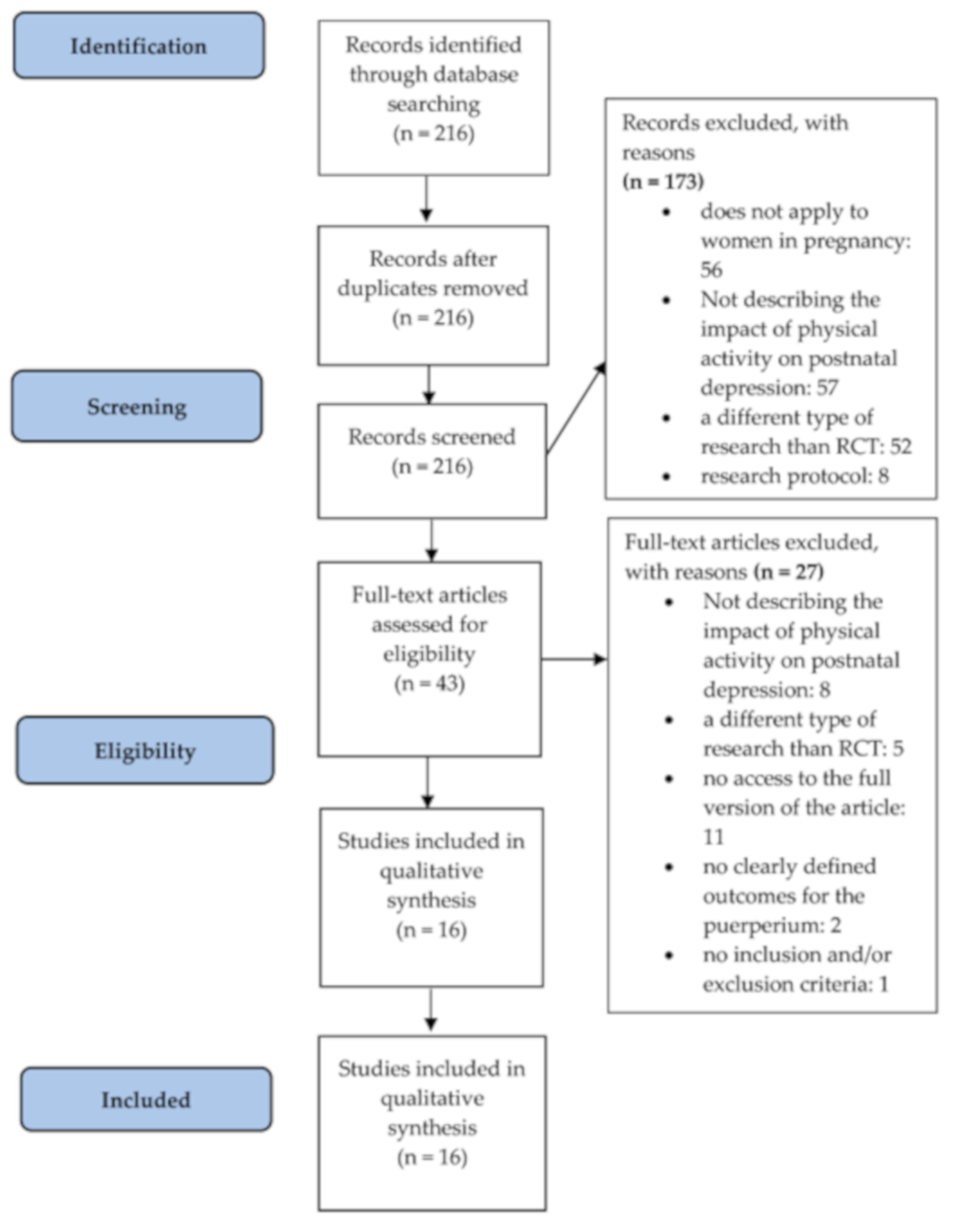 Medicina Free Full Text Physical Activity And The Occurrence Of Postnatal Depression A Systematic Review Html
Medicina Free Full Text Physical Activity And The Occurrence Of Postnatal Depression A Systematic Review Html
 Fda Approves First Treatment For Postpartum Depression
Fda Approves First Treatment For Postpartum Depression
 Treatment Of Mild To Moderate Postpartum Depression During Breastfeeding Download Table
Treatment Of Mild To Moderate Postpartum Depression During Breastfeeding Download Table
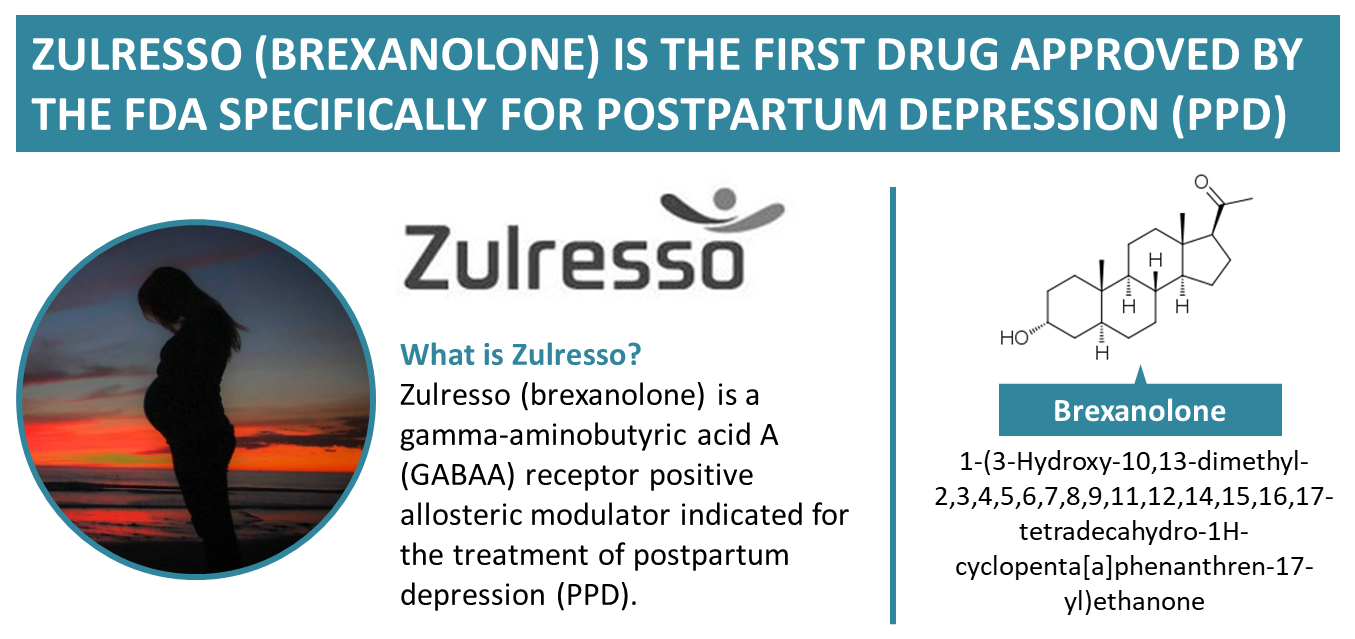 Zulresso Lawsuits Zulresso Settlements Consumer Alert Now
Zulresso Lawsuits Zulresso Settlements Consumer Alert Now
No comments:
Post a Comment
Note: Only a member of this blog may post a comment.