This is the largest clinical trial evaluating a PD-1 inhibitor as a first-line monotherapy in patients with advanced non-small cell lung cancer with high PD-L1 expression. January 10 2020 Key Record Dates.
 Rebuild Clinical Trials In The Image Of Patient Need Join Sanofi Janssen Fda Pfizer And More Medcity News
Rebuild Clinical Trials In The Image Of Patient Need Join Sanofi Janssen Fda Pfizer And More Medcity News
12032021 - Sanofi and Translate Bio initiate Phase 12 clinical trial of mRNA COVID-19 vaccine candidate Clinical trial to assess safety immune response and reactogenicity after preclinical.

Sanofi clinical trials. 13 Zeilen Clinical Study Results Sanofi Pharma including Sanofi Genzyme. NCT02906020 Other Study ID Numbers. Sanofi Pasteur a Sanofi Company.
Sanofi which is developing. September 3 2020 Key Record. Genzyme a Sanofi Company.
NCT01427309 Other Study ID Numbers. Without clinical trials new medicine may never make it from the research lab to patients in need. WHO First Posted.
NCT02782741 Other Study ID Numbers. VAT00001 U1111-1250-4757 Other Identifier. Trial Transparency email recommended Toll free number for US Canada 800-633-1610 ext option 6.
WHO universal trial number First Posted. May 25 2016 Key Record Dates. April 5 2021 Last Verified.
These carefully designed studies can provide important data that include proper dosage benefit to. Sanofi GSK keep up vaccine development efforts. HSV15 U1111-1183-6522 Other Identifier.
UTN First Posted. Genzyme a Sanofi Company. The positive results are extremely encouraging and we look forward to advancing a potential new treatment option for these patients said John Reed MD PhD Global Head of Research and Development at Sanofi.
NCT04537208 Other Study ID Numbers. UTN First Posted. Sanofi Pasteur a Sanofi Company.
EFC14028 2016-000942-77 EudraCT Number U1111-1178-4806 Other Identifier. ACT14820 2016-000657-12 EudraCT Number U1111-1180-6918 Other Identifier. PARIS September 1 2020 Sanofi today announced that the global Phase 3 trial investigating intravenously administered Kevzara sarilumab at a dose of 200 mg or 400 mg a in severely or critically ill b patients hospitalized with COVID-19 did not meet its primary endpoint and key secondary endpoint c when Kevzara was compared to placebo added to usual hospital care.
September 19 2016 Key Record Dates. Sanofi Pasteur a Sanofi Company. September 1 2011 Key Record Dates.
April 20 2015 Last Verified. Sanofi said in a statement Friday it would be recruiting 415 participants for a Phase III clinical trial to assess the shots safety side effect profile and dosing. FIM12 U1111-1120-1300 Other Identifier.
Britains GlaxoSmithKline and Frances Sanofi on Monday said they had started a new clinical trial of their protein-based Covid-19 vaccine candidate. NCT04222985 Other Study ID Numbers. Last month Sanofi and GlaxoSmithKline launched a new clinical trial of its first COVID-19 vaccine candidate.
UTN First Posted. Sanofis Digital Initiative Simplifies Patient Participation in Clinical Trials.
 Clinical Trials And Results Sanofi Uk Sanofi In The United Kingdom
Clinical Trials And Results Sanofi Uk Sanofi In The United Kingdom
 Clinical Trials Results Sanofi
Clinical Trials Results Sanofi
 Sanofi Improves Clinical Trial Monitoring With N Side Suite For Clinical Trials
Sanofi Improves Clinical Trial Monitoring With N Side Suite For Clinical Trials
 Sanofi Trinetx Partner To Optimize Clinical Trials With Digital Technology
Sanofi Trinetx Partner To Optimize Clinical Trials With Digital Technology
 Sanofi Announces Clinical Trial Of Second Covid 19 Vaccine Candidate
Sanofi Announces Clinical Trial Of Second Covid 19 Vaccine Candidate
 Sanofi Gsk To Start Potential Covid 19 Vaccine Trial
Sanofi Gsk To Start Potential Covid 19 Vaccine Trial
 What Clinical Trial Results Now You Can See Who Isn T Sharing Their Findings
What Clinical Trial Results Now You Can See Who Isn T Sharing Their Findings
 Sanofi Improves Clinical Trial Monitoring With N Side Suite For Clinical Trials
Sanofi Improves Clinical Trial Monitoring With N Side Suite For Clinical Trials
 Clinical Trials Can Make Anyone A Hero Sanofi
Clinical Trials Can Make Anyone A Hero Sanofi
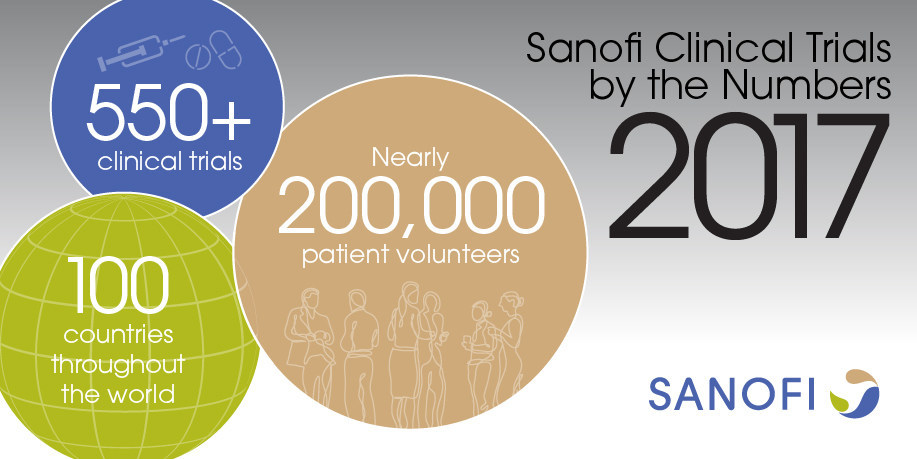 Sanofi Accelerating Future Breakthroughs Through Clinical Trials Part 1 Of 3
Sanofi Accelerating Future Breakthroughs Through Clinical Trials Part 1 Of 3
 Sanofi Gsk Announce Setback In Clinical Trial Europe News Top Stories The Straits Times
Sanofi Gsk Announce Setback In Clinical Trial Europe News Top Stories The Straits Times
![]() Sanofi S Digital Initiative Simplifies Patient Participation In Clinical Trials Sanofi
Sanofi S Digital Initiative Simplifies Patient Participation In Clinical Trials Sanofi
 Parexel Sanofi Partner To Advance Wearable Tech In Clinical Trials
Parexel Sanofi Partner To Advance Wearable Tech In Clinical Trials
 Optimizing Clinical Trials With Digital Technology Sanofi
Optimizing Clinical Trials With Digital Technology Sanofi
No comments:
Post a Comment
Note: Only a member of this blog may post a comment.