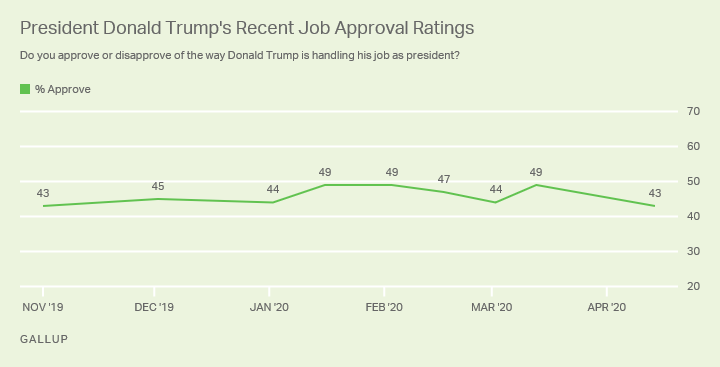
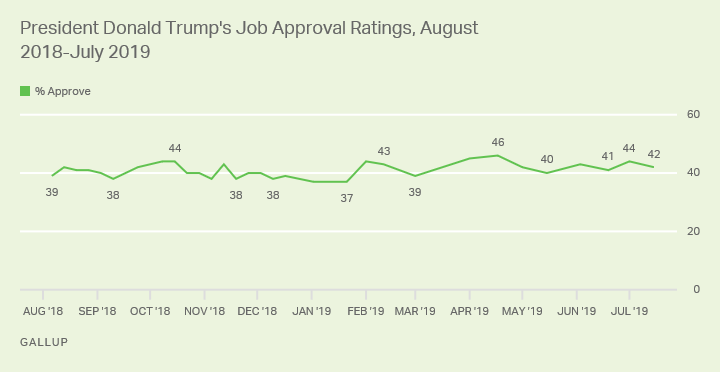
The Prescription Drug User Fee Act PDUFA goal date for the FDA to make a decision on the tanezumab application is in December 2020. Potential FDA approval for tanezumab still looked rocky as the regulator casts doubt over its safety.
 Fda Starts Review Of Pfizer Lilly S Non Opioid Painkiller
Fda Starts Review Of Pfizer Lilly S Non Opioid Painkiller
Tanezumab a so-called monoclonal antibody appears to work by blocking nerve growth factor a protein involved in regulating nerve cells.

Tanezumab fda approval. While we are disappointed with todays outcome we continue to believe that tanezumab has a positive benefit-risk profile for patients with moderate-to. In 2009 there was a Phase III trial for knee pain due to osteoarthritis OA. In its acceptance letter the FDA stated that it is currently planning to hold an Advisory Committee meeting to discuss this application.
PFE along with partner Eli Lilly LLY announced that the FDA Joint Arthritis Advisory Committee and Drug Safety and Risk Management Advisory Committee have. 8 rijen Tanezumab FDA Approval Status. Tanezumab is an investigational monoclonal antibody in a new class of medicines called nerve growth factor NGF inhibitors which work in a different manner than currently available treatments such as opioids nonsteroidal anti-inflammatory drugs NSAIDs and other analgesics.
Pfizer said it would continue to pursue approval for tanezumab. If approved the companies will jointly commercialize tanezumab in the US. And Pfizer will be responsible for commercialization activities outside of the US.
Pfizer and Lilly are driven by our shared mission to improve the lives of the millions of people who are suffering from moderate-to-severe OA pain and. Pfizers tanezumab development team leader Ken Verburg said. On the question of will the REMS proposed by.
Tanezumab is a humanized monoclonal antibody that targets nerve growth factor NGF a protein that increases in the body because of injury inflammation or chronic pain. Tanezumab was discovered and developed by Rinat Neuroscience and was acquired by Pfizer in 2006. An FDA expert panel has turned down a recommendation to approve tanezumab to treat signs and symptoms of moderate to severe osteoarthritis in adults.
While we are disappointed in todays outcome. Then the FDA will take it under advisement and come to its own decision. Whether the FDA eventually decides to greenlight the drug comes after 15 years and 40 trials worth of data testing.
Tanezumab binds to NGF and inhibits pain signals from muscles skin and organs from reaching the brain. In 2013 Pfizer and Lilly entered into a collaboration to develop and commercialize tanezumab. FDA panels reject tanezumab to treat arthritis pain.
Link is external and Eli. FDA Accepts Regulatory Submission for Tanezumab a Potential First-in-Class Treatment for Patients with Chronic Pain Due to Moderate-to-Severe Osteoarthritis. The Prescription Drug User Fee Act PDUFA goal date for the FDA to make a decision on the tanezumab application is in December 2020.
Blocking the growth factor prevents stimulation of. The primary safety worry is rapidly progressing osteoarthritis dubbed as. In its acceptance letter the FDA stated that it is.
Tanezumab INN codenamed RN624 is a monoclonal antibody against nerve growth factor as a treatment for pain. The FDA is not obligated to follow the panels recommendation but the almost unanimous vote against the drugs safety profile makes US approval unlikely. The FDA set December 2020 as a goal for making a decision on the application.
Long-term use of tanezumab resulted in moderate improvements in pain and function and demonstrated a safety profile comparable to NSAIDs and opioids. Monday March 02 2020 - 0145am. The safety questions at hand deal with reports of unusual and unexpected joint-related adverse events in tanezumab-treated patients with osteoarthritis FDA explained noting.
Outside experts advising the FDA voted 19 to 1 against Pfizers potential osteoarthritis drug tanezumab saying the proposed risk evaluation and mitigation strategy REMS will.